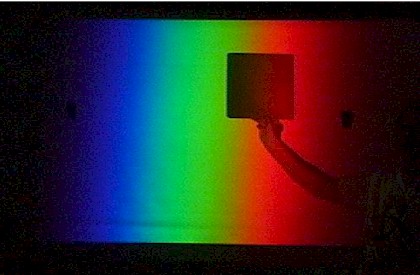
Demonstrations With Absorption Spectra
INTRODUCTION:
Dr. Philip Sadler in The Physics Teacher
(Oct. 1991) described research he had carried out on students'
understanding of the principles that govern colour perception.
His research indicated that light and colour misconceptions
extend from student age right through to people training to be
teachers.
Some very common misconceptions that can be
addressed by this demonstration are:
1. Filters change one colour of light into another colour.
2. Coloured lights combine the same way that paints do (mixing
red and green will produce brown, not yellow)
3. The colour of an object is independent of the light that
illuminates it (a red apple is still red even if you shine blue
light on it!)
In the article, he described using an overhead projector and a
holographic diffraction grating to produce a simple spectrum.
This demonstration extends his ideas somewhat.
CHEMICAL CONCEPTS:
-colour reflection and absorption
-fluorescence
-acid/base colour indicators
MATERIALS:
-overhead projector and screen
-holographic diffraction grating
- 3 small mirrors fixed to retort stands
-cardboard cover for stage of the projector with a 1 cm slit cut
in the centre
-red paper
-green solution in a flat-sided bottle
-white paper with FRESH markings from a yellow highlighter marker
-150-mL beaker
-water
-dropper bottle of 1% phenolphthalein solution
-dropper bottles of dilute (0.1M) NaOH and HCl solutions
-stirring rod
PROCEDURE and DISCUSSION:
Tape a holographic diffraction grating film on the lens of the overhead projector (or on the tilting mirror) so the grating is hanging vertically. Place a cardboard screen on the stage to form a 1 cm wide slit about 12 cm long. Focus the image of the slit on the screen and rotate the projector so the white image is just on the edge of the screen. A bright visible spectrum will be projected on the middle of the screen.
(a) additive colour mixing:
Have three students position the mirrors fixed to the retort stands so that each student intercepts a different colour on the screen (red, green and blue). Since the emitted blue is less intense than the green or red, have the "blue" student stand closest to the projected beam and have the others position themselves behind. The students should then rotate the stands so that the images from their mirrors overlap on a white section of wall. Red and green will form an obvious yellow. When blue is added the light will appear white.
(b) colour by selective absorption:
Stand close to the screen and hold a piece of red paper in the white image of the slit on the side of the screen. Ask for student predictions about its appearance in each colour region. Begin in the red region and slowly slide it across the screen. It changes from bright red to black when it enters the green region. It looks black in blue light as well. The dye in the paper absorbs green and blue light so it will only look red (ie reflect red light to their eyes) when there is red light shining on the page.

Stand close to the screen and hold a
flat-sided bottle filled with green liquid in the white
image of the slit. Again, get predictions of its appearance in
each region based on the behavior of the red paper.
Begin in the red region. The bottle will appear black and opaque.
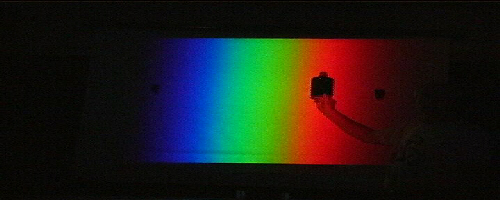
Move to the green region and the bottle will become transparent. It does not absorb green light… so the green light passes through the bottle in both directions!

The bottle regains its black opaque appearance in the blue
region.
[Note: the bottle must have flat sides. A curved container will act like a lens and focus whatever light gets through the dye into a bright strip on the screen behind. This is an interesting effect, but not useful here.]
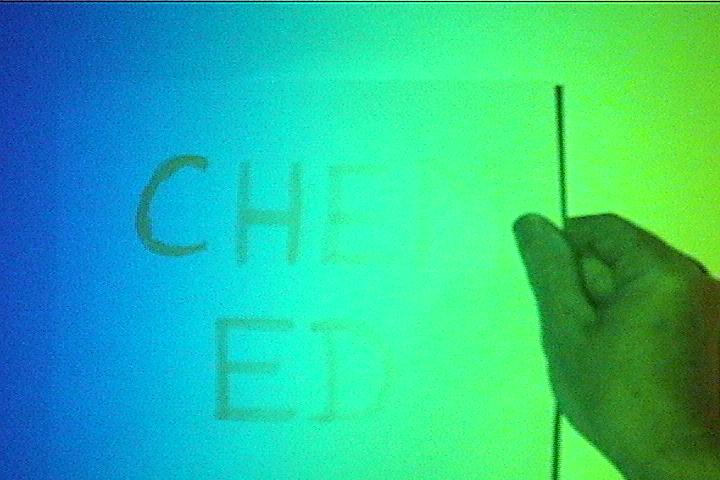
(c) colour with invisible light:
Have a white sheet of paper with large letters written on it with yellow highlighter. (Fresh writing works best.) Start in the red end of the spectrum and slowly slide the paper across the screen. The page will appear blank until it gets into the blue region where the letters will start to fluoresce. The fluorescence will continue as you move beyond the edge of the blue region, showing that the projector bulb is producing invisible UV light. The ink is turning that energy into bright yellow light.

(d) how acid base indicators change colour: (a live action demo)
Add about 50 mL of water and a few drops of phenolphthalein dye to a 150-mL beaker and place the beaker on the middle of the cardboard slit. There should be unobstructed spectrum strips above and below the beaker image to act as references.
Use the stirring stick to start the solution slowly rotating in the beaker. Then add a drop or two of NaOH solution. As the pink clouds of basic dye drift over the slit (and into the "white" image on the side of the screen), green will dramatically disappear from the spectrum. When the dye has completely turned purple, there will be a dark patch where the green light used to be on the screen.

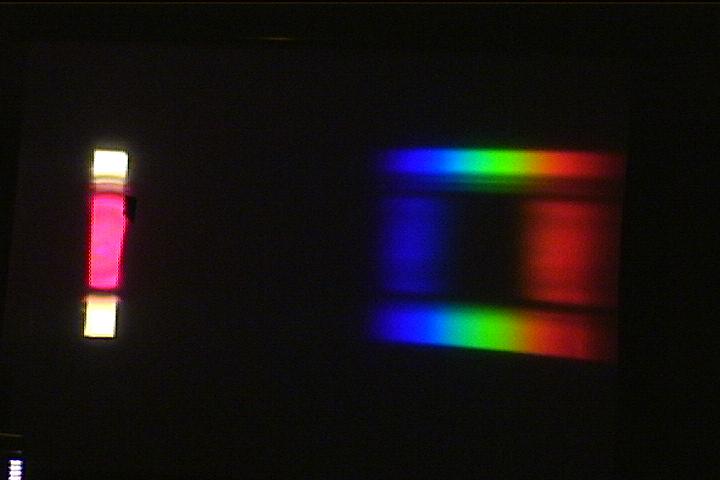
It is a dramatic way to show that the
purple colour arises when green light is absorbed in the
solution.
Drops of HCl solution can restore the solution to colourless and
the green patch reappears on the screen.
[Bromthymol dye is also effective. When the dye is yellow, there is no blue light passing through the solution and onto the screen. When the dye turns blue, only green and blue light pass through. Red is absorbed by the base form of the dye.]
SAFETY PRECAUTIONS:
Tape the edges of the mirrors so students
will not cut their fingers while aiming the stands.
Use standard precautions with bottles of dilute acids and bases.
All chemical materials are dilute enough to be washed down the
sink.
DISPOSAL:
All solutions used here are dilute enough to be washed down
the sink.
ACKNOWLEDGMENT:
The Physics Teacher: Philip M. Sadler. Projecting Spectra for Classroom Investigations. The Physics Teacher, 29(7), 1991, pp. 423-427
Materials available from Flinn
Scientific:
Catalog No. Description
AP6172 Color and Light Spectrum
Demonstrations Kit
....... (This
kit has everything you need for these demos... except the
overhead projector!)
P0019 Phenolphthalein solution 1%
S0149 Sodium Hydroxide solution 0.1M
H0014 Hydrochloric acid solution 0.1M